7-Dehydrocholesterol

| |
| |
| Names | |
|---|---|
| IUPAC name
Cholesta-5,7-dien-3β-ol
| |
| Systematic IUPAC name
(1R,3aR,7S,9aR,9bS,11aR)-9a,11a-Dimethyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,6,7,8,9,9a,9b,10,11,11a-dodecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.456 |
| MeSH | 7-dehydrocholesterol |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H44O | |
| Molar mass | 384.638 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

7-Dehydrocholesterol (7-DHC) is a zoosterol that functions in the serum as a cholesterol precursor, and is photochemically converted to vitamin D3 in the skin, therefore functioning as provitamin-D3. The presence of this compound in human skin enables humans to manufacture vitamin D3 (cholecalciferol). Upon exposure to ultraviolet UV-B rays in the sun light, 7-DHC is converted into vitamin D3 via previtamin D3 as an intermediate isomer. It is also found in the milk of several mammalian species.[1][2] Lanolin, a waxy substance that is naturally secreted by wool-bearing mammals, contains 7-DHC which is converted into vitamin D by sunlight and then ingested during grooming as a nutrient. In insects 7-dehydrocholesterol is a precursor for the hormone ecdysone, required for reaching adulthood.[3] 7-DHC was discovered by Nobel-laureate organic chemist Adolf Windaus.
Biosynthesis
[edit]It is synthesized from lathosterol by the enzyme lathosterol oxidase (lathosterol 5-desaturase). This is the next-to-last step of cholesterol biosynthesis.[4] Defective synthesis results in the human inherited disorder lathosterolosis resembling Smith–Lemli–Opitz syndrome.[4] Mice where this gene has been deleted lose the ability to increase vitamin D3 in the blood following UV exposure of the skin.[5]
Location
[edit]The skin consists of two primary layers: an inner layer, the dermis, comprising largely connective tissue, and an outer, thinner epidermis. The thickness of the epidermis ranges from 0.08 mm to greater than 0.6 mm (from 0.003 to 0.024 inches).[6] The epidermis comprises five strata; from outer to inner, they are the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. The highest concentrations of 7-dehydrocholesterol are found in the epidermal layer of skin—specifically in the stratum basale and stratum spinosum.[7] The production of pre-vitamin D3 is, therefore, greatest in these two layers.
Radiation
[edit]Synthesis of pre-vitamin D3 in the skin involves UVB radiation, which effectively penetrates only the epidermal layers of skin. 7-Dehydrocholesterol absorbs UV light most effectively at wavelengths between 295 and 300 nm and, thus, the production of vitamin D3 will occur primarily at those wavelengths.[8] The two most important factors that govern the generation of pre-vitamin D3 are the quantity (intensity) and quality (appropriate wavelength) of the UVB irradiation reaching the 7-dehydrocholesterol deep in the stratum basale and stratum spinosum.[7] Light-emitting diodes (LEDs) can be used to produce the radiation.[9]
Another important consideration is the quantity of 7-dehydrocholesterol present in the skin. Under normal circumstances, ample quantities of 7-dehydrocholesterol (about 25–50 μg/cm2 of skin) are available in the stratum spinosum and stratum basale of human skin to meet the body's vitamin D requirements. 7-DHC insufficiency has been proposed as an alternate cause for Vitamin D deficiency.[10]
Sources
[edit]7-DHC can be produced by animals and plants via different pathways. It is not produced by fungi in significant amounts. It is made by some algae, but the pathway is poorly understood.[11]
Industrially, 7-DHC generally comes from lanolin, and is used to produce vitamin D3 by UV exposure.[12] Lichen (Cladonia rangiferina) is used to produce vegan D3.[13][14]
7-DHC is used for vitamin D3 synthesis via lanosterol in land animals, via cycloartenol in plants, and in algae together with another provitamin D ergosterol for D2. In fungi solely ergosterol is used for synthesis of D2 via lanosterol.[15]
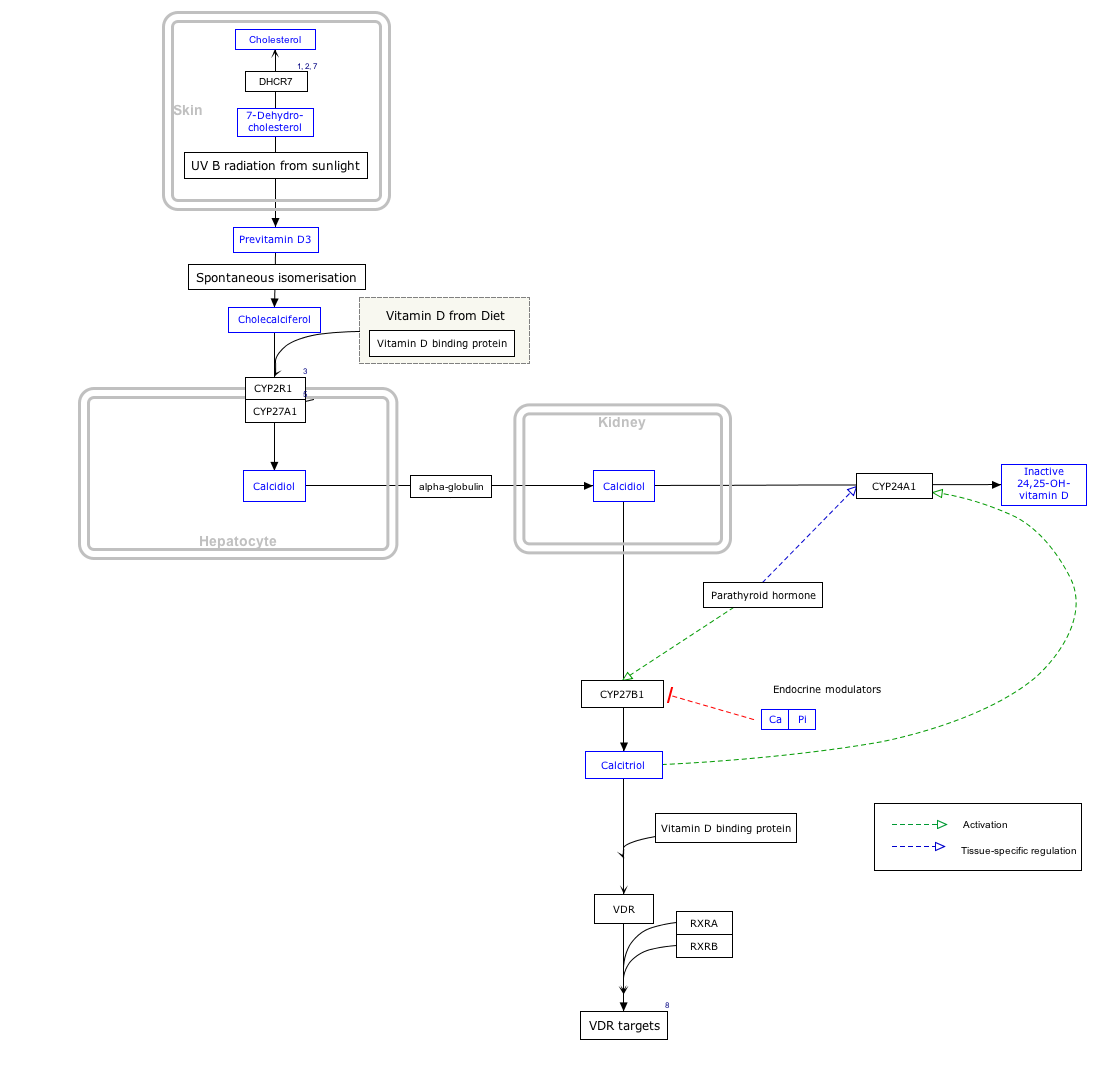
Interactive pathway map
[edit]Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
See also
[edit]References
[edit]- ^ "7-dehydrocholesterol". The American Heritage Stedman's Medical Dictionary. Houghton Mifflin Company. 21 January 2007.
- ^ "7-dehydrocholesterol". Answers.com. Archived from the original on 25 October 2012.
- ^ Young E (2012). "Thanks to one gene, this fly needs a cactus to escape Neverland". Not Exactly Rocket Science. Archived from the original on 2012-09-30. Retrieved 2012-09-28.
- ^ a b Krakowiak PA, Wassif CA, Kratz L, Cozma D, Kovárová M, Harris G, Grinberg A, Yang Y, Hunter AG, Tsokos M, Kelley RI, Porter FD (July 2003). "Lathosterolosis: an inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency". Human Molecular Genetics. 12 (13): 1631–41. doi:10.1093/hmg/ddg172. PMID 12812989.
- ^ Makarova AM, Pasta S, Watson G, Shackleton C, Epstein EH (July 2017). "Attenuation of UVR-induced vitamin D3 synthesis in a mouse model deleted for keratinocyte lathosterol 5-desaturase". The Journal of Steroid Biochemistry and Molecular Biology. 171: 187–194. doi:10.1016/j.jsbmb.2017.03.017. PMID 28330720. S2CID 206502190.
- ^ Martini F, Timmons MJ, Tallitsch RB (2006). Human Anatomy. Pearson/Benjamin-Cummings Publishers. p. 89. ISBN 0-8053-7211-3.
- ^ a b Norman AW (June 1998). "Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system". The American Journal of Clinical Nutrition. 67 (6): 1108–1110. doi:10.1093/ajcn/67.6.1108. PMID 9625080.
- ^ MacLaughlin JA, Anderson RR, Holick MF (May 1982). "Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin". Science. 216 (4549): 1001–3. doi:10.1126/science.6281884. PMID 6281884.
- ^ Kalajian TA, Aldoukhi A, Veronikis AJ, Persons K, Holick MF (September 2017). "Ultraviolet B Light Emitting Diodes (LEDs) Are More Efficient and Effective in Producing Vitamin D3 in Human Skin Compared to Natural Sunlight". Scientific Reports. 7 (1): 11489. doi:10.1038/s41598-017-11362-2. PMC 5597604. PMID 28904394.
- ^ Gokhale S, Bhaduri A (December 2019). "Provitamin D3 modulation through prebiotics supplementation: simulation based assessment". Scientific Reports. 9 (1): 19267. Bibcode:2019NatSR...919267G. doi:10.1038/s41598-019-55699-2. PMC 6917722. PMID 31848400.
- ^ Jäpelt RB, Jakobsen J (2013). "Vitamin D in plants: a review of occurrence, analysis, and biosynthesis". Frontiers in Plant Science. 4: 136. doi:10.3389/fpls.2013.00136. PMC 3651966. PMID 23717318.
- ^ Holick MF (November 2005). "The vitamin D epidemic and its health consequences". The Journal of Nutrition. 135 (11): 2739S–2748S. doi:10.1093/jn/135.11.2739S. PMID 16251641.
[Vitamin D3] is produced commercially by extracting 7-dehydrocholesterol from wool fat, followed by UVB irradiation and purification [...] [Vitamin D2] is commercially made by irradiating and then purifying the ergosterol extracted from yeast
- ^ "Vitamin D". The Vegan Society.
- ^ Gangwar, Gourvendra (1 July 2023). "Formulation of Lichen Based Pill a Natural Source of Vitamin D3 with a High Absorption Rate by Ambrosiya Neo-Medicine Pvt. Ltd". International Journal of Biomedical Investigation: 1.
- ^ Göring, Horst (November 2018). "Vitamin D in Nature: A Product of Synthesis and/or Degradation of Cell Membrane Components". Biokhimiya (Moscow). 83 (11): 1350–1357. doi:10.1134/S0006297918110056. PMID 30482146. S2CID 53437216. Retrieved December 2, 2023.